EPJ E Highlight - Electrical disorder acts like a traffic light for a biological gate
- Details
- Published on 30 November -0001
New study of how positive and negative electrical charge disorder at the ends of polymers acts like a green or red light for proteins to pass through biological membranes
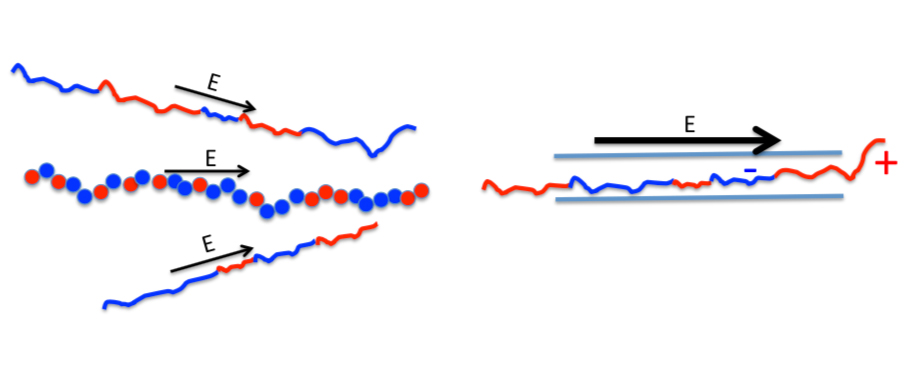
Nature’s way of allowing proteins across its gates, through porous biological membranes, depends, among others, on their electrical charge. For a protein to cross this type of membrane, it needs to be stimulated by an electrical field. A new study focuses on a particular kind of proteins that have multiple functions - dubbed Intrinsically Disordered Proteins - because the electric charge disorder on their surface makes it possible for them to take multiple shapes. In the work, recently published in EPJ E, Albert Johner from the Charles Sadron Institute (part of the CNRS) in Strasbourg, France and Jean-Francois Joanny from Paris reveal how the mixed electrical charge at the ends of the proteins influences biological membrane crossing. This has potential implications for our understanding of how proteins travel across the body, and of disease mechanisms.
Physicists studying protein membrane crossing often employ a simplified model made of polymers carrying positive and negative electrical charges. Typically, the attraction between opposite charges, scattered randomly throughout the chain, makes the molecule shrink into a dense tangle of polymer fibres. Plunginthese polymersrs in a solution with a high concentration of salt reduces the electrical attraction and causes the polymer to expand.
In this study, the authors examine the role of disorder in the charge distribution along the polymer chain. They establish the direction in which the polymer chain engages in the pore of the membrane. They also look at how long a typical polymer is blocked at the membrane’s gate. And they look at the speed with which polymers can cross this biological gate for two specific Intrinsically Disordered Proteins that differ in their length and structure. The authors find that, for one specific protein, crossing prevails over rejection if the protein starts at one end, and that rejection is more likely than crossing if the protein starts at the other end.
Translocation of Polyampholytes and Intrinsically Disordered Proteins. A. Johner and J.F. Joanny (2018), Eur. Phys. J. E 41: 78, DOI 10.1140/epje/i2018-11686-7





