EPJ E Highlight - How lactoferrin clamps down on free roaming iron ions to stop nefarious effects on cells
- Details
- Published on 20 September 2018
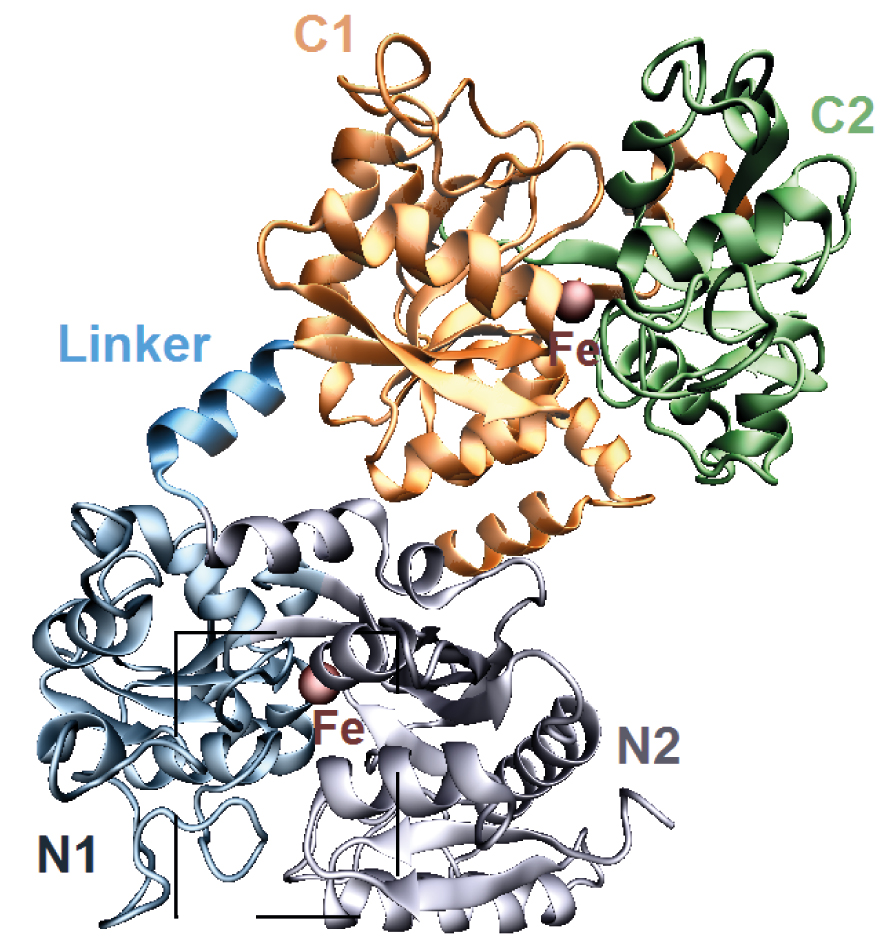
New study elucidates structure of the protein lactoferrin as it undergoes transition from an open to a closed structure to decrease the level of free iron ions in the body
What prevents our cells from being overexposed to iron ions roaming freely in the body is a protein called lactoferrin, known for its ability to bind tightly to such ions. These free ions are essential for a number of biological processes. If found in excessive quantities, however, they could cause damage to proteins and DNA in the body, sometimes even leading to cell death. This is because free iron ions lead to an increase of the concentration of reactive substances with oxidising power roaming freely in the body. This has driven scientists to develop a better understanding of how lactoferrin's structural change helps to clamp down on free iron ions. In a new study published in EPJ E, Lilia Anghel from the Institute of Chemistry in Chisinau, Republic of Moldova, and research collaborators study the changes in the structure of lactoferrin as it binds to iron ions, using combined experimental and molecular dynamics simulations.
Scientists who have previously studied the X-ray crystal structure of human lactoferrin have shown that changes in conformation within the protein structure occur as the iron ion binds to it. In this study, the authors rely on a method called small angle neutron scattering to detect the structural differences between the open and closed conformation of human lactoferrin in solution.
The authors demonstrate that an amino acid - namely Arginine 121 - plays a key role in the conformation stability of the lactoferrin protein. In addition, by focusing on understanding how human lactoferrin changes from its open to its closed conformation, they also find that the open conformation appears to offer a smaller twisting radius than that of the closed version.
Lastly, they detect visible differences between the two low-resolution, three-dimensional models of open and closed structure of human lactoferrin in solution. Both have a more compact conformation than high-resolution structures.
L. Anghel, A. Radulescu, R. V. Erhan (2018), Structural aspects of human lactoferrin in the iron binding process studied by molecular dynamics and small-angle neutron scattering,
Eur. Phys. J. E, 2018, 41:109. DOI 10.1140/epje/i2018-11720-x




